Analyzing polar compounds via high-performance liquid chromatography (HPLC) has long posed a challenge due to their limited retention on reverse-phase stationary phases, the most widely used in laboratories. To increase the retention of these compounds in reverse phase, ion-pairing chromatography or derivatization has been employed to decrease their polarity. Alternative stationary phases with charged functional groups (ion-exchange chromatography) or even normal-phase HPLC have also been explored.
Recently, Hydrophilic Interaction Chromatography (HILIC) has gained traction as an efficient and cost-effective alternative for HPLC’s analysis of such molecules, offering significant cost reductions and improved separation efficiency. Importantly, this technique enables the separation of highly polar compounds using the same systems and solvents as reverse-phase HPLC (RPLC). However, despite the advantages of HILIC for challenging separations, the technique faces issues such as poor reproducibility and sample or additive solubility challenges in mobile phases with high organic content. Developing HILIC analytical methods, therefore, requires a thorough understanding of the technique and its key differences from reverse-phase chromatography.
HILIC: a growing technique
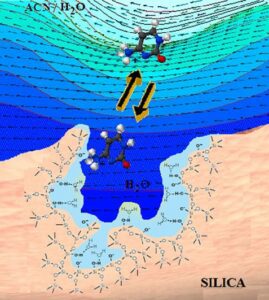
Hydrophilic Interaction Chromatography differs fundamentally from reverse-phase liquid chromatography in its use of a polar, hydrophilic stationary phase. The mobile phase, in contrast, is highly organic (typically over 60% solvent, often acetonitrile) and contains a small percentage of aqueous solvent or buffer, as well as any other polar solvent. This combination allows the mobile phase to form a water-rich layer adsorbed onto the polar surface of the stationary phase.
In this environment, polar analytes—substances with an affinity for water—will partition within this stationary-phase layer, achieving retention through a combination of mechanisms:
- Liquid-liquid partitioning: Analytes distribute between the water-rich layer and the predominantly organic mobile phase, depending on their affinity for each phase.
- Hydrogen bonding: Polar functional groups in analytes may form hydrogen bonds with the stationary phase, contributing to retention.
- Electrostatic interactions: Charged functional groups interact with oppositely charged sites on the stationary phase, aiding analyte retention.
- Van der Waals interactions: Under optimal experimental conditions, Van der Waals forces may occur between hydrophobic portions of ligands bound to the stationary phase (or siloxane groups in low organic solvent content) and the non-polar regions of analytes. These weak, additional interactions work alongside stronger mechanisms to ensure analytes remain retained in the stationary phase.
Implementation and advantages:
To implement HILIC effectively, the proper column selection is crucial. Since polarity and hydrophilicity of the stationary phase must be ensured, selecting among various available stationary phases (e.g., silica, zwitterionic, amide, aminopropyl) will influence the method’s selectivity, and reviewing prior studies can limit the need for extensive “trial-and-error” phases.
Choosing an appropriate mobile phase is also essential. In this case, water is the strong solvent, and the weak solvent is organic and must be water-miscible. Acetonitrile (ACN) is the most common choice, although methanol or alcohols may be added to improve selectivity for certain applications.
Another significant advantage of HILIC is the low backpressure resulting from the low viscosity of organic-rich mobile phases, leading to faster analysis times. This is particularly beneficial when using columns with particle sizes below 2 micrometers, as lower backpressure enables high flow rates. Additionally, frictional heating, which can be problematic with small particles in reverse-phase conditions, is generally not an issue in HILIC due to the pressure being 2 to 3 times lower.
HILIC also allows for direct injection of aprotic organic solvent extracts obtained via solid-phase extraction cleanup protocols, without the need for drying and reconstitution in the mobile phase. Additionally, HILIC enables the separation of cations, anions, and neutral polar compounds in a single injection, offering mass spectrometry performance equal to or exceeding that of reverse-phase liquid chromatography.
Applying HILIC efficiently
Despite its benefits, Hydrophilic Interaction Liquid Chromatography (HILIC) presents specific challenges that can affect its performance, such as reproducibility and re-equilibration issues. Each of these challenges can, however, be resolved with proper adjustment:
- Problem: Slow re-equilibration:
The primary cause is often insufficient water in the mobile phase, as this phase must contain enough aqueous substance to renew the surface layer after each run. To ensure rapid re-equilibration, the column should never operate with a mobile phase containing less than 5% water. - Problem: Insufficient reproducibility
Reproducibility issues can stem from three main causes. If the issue originates from incorrect re-equilibration prior to the next injection, ensure the mobile phase contains sufficient water to renew the surface layer rapidly. If re-equilibration is incomplete, extend the re-equilibration time.
If reproducibility issues relate to interaction with active sites on stainless steel, it is ideal to use equipment designed and manufactured with special materials to reduce these non-specific steel interactions, such as the Waters Bio/Premier ranges. Additionally, if the sample solvent is too strong (i.e., high water or alcohol content), increasing the organic content of the sample solvents and/or reducing injection volumes can help maintain retention consistency. If the issue relates to interaction with active sites in the system’s stainless steel, washing with phosphoric or nitric acid or switching to an inert HPLC system may be necessary to minimize nonspecific interactions, particularly common with biomolecules.
- Problem: Sample solubility
Sample dissolution issues often arise with organic solvents that are ideal for HILIC chromatography but may be poor solvents for salts and other polar analytes. In such cases, diluting samples in acetonitrile as much as solubility allows, and then reducing injection volumes, can help achieve a satisfactory peak shape with good reproducibility. - Problem: Peak shape
Several primary factors can impact peak shape. Peaks may split or tail due to excessive strong solvent (water) volume in the system. Diluting the sample in a weaker solvent, such as acetonitrile, can address this issue.
Another potential cause is sample adsorption onto active sites within the stainless steel system. If the analyte is particularly “sticky,” system washing and inert hardware may be necessary. Additionally, certain HILIC phases, such as silica, can exhibit secondary ion-exchange interactions, leading to excessive retention or trailing of anions or cations. Switching to interlaced phases without these secondary interactions can mitigate this.
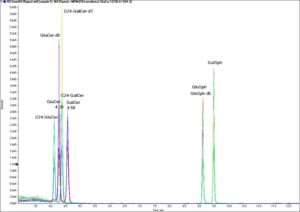
Comparison of three different HILIC columns. Problems with peak shape can contaminate information on separation performance, missing potential problems in the chromatographic system. Source: Sommella, Eduardo & Salviati, Emanuela & Musella, Simona & Di Sarno, Veronica & Gasparrini, Francesco & Campiglia, Pietro. (2020). Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics. Separations. 7. 25. 10.3390/separations7020025. Finally, peak shape issues can arise from low mobile-phase buffer concentration. While increasing buffer concentration may improve peak shape, it may also impact mass spectrometry sensitivity (MS) and baseline stability in ELSD and UV detection.
- Problem: Buffer solubility
Buffer solubility issues often relate to the high organic solvent content needed for HILIC retention of less polar analytes, as many buffers are poorly soluble. Adding a small amount of water can significantly enhance buffer solubility in acetonitrile. Specifically, adding 10% water is recommended, and solubility testing with high-concentration buffer/organic solvent mixtures should be performed.
HILIC at AMSbiopharma: from Sphingolipids to Glycans
While Hydrophilic Interaction Chromatography is not yet a universal technique, AMS Biopharma applies HILIC-based methods for analyzing polar biomolecules. This approach is particularly relevant for lipidomics and metabolite analysis in metabolomic studies when coupled with mass spectrometry.
Our experience in developing and validating HILIC methods enables us to conduct cutting-edge analytical studies with exceptional efficiency in chromatographic separation, even for structurally similar biomolecules—common in biomarker studies.