Semaglutide has emerged as a groundbreaking therapy for managing type 2 diabetes, marketed under brand names like Ozempic. As a glucagon-like peptide-1 (GLP-1) receptor agonist, it significantly benefits glucose regulation and weight management.
Its patent is set to expire in 2026 in countries like China, paving the way for generic alternatives. Currently, at least 15 Chinese pharmaceutical companies are actively developing generic versions, with more expected to follow around the world as patents expire. This growing competition underscores the importance of strict controls of Ozempic impurities to ensure the safety and efficacy of these alternatives.
What Does Ozempic Contain? Breaking Down Semaglutide’s Composition and Impurities
Ozempic’s active pharmaceutical ingredient (API) is semaglutide, a long-acting human GLP-1 receptor agonist, which specifically activates the GLP-1 receptor (GLP-1R). Semaglutide is a therapeutic peptide produced by recombinant DNA (rDNA) technology in genetically engineered yeast cells. It shares a 94% structural homology with native GLP-1, however, semaglutide contains key modifications to enhance its stability and effectiveness.
The first six amino acids at the N-terminus are removed, and the alanine at position 8 is replaced with Aib (2-aminoisobutyric acid) to prevent enzymatic breakdown by endogenous enzymes in vivo. Additionally, the lysine at position 28 is substituted with arginine, leaving the lysine at position 26 available for attachment to a fatty-acid/ethylene glycol-like chain, which extends the drug’s half-life by binding to serum albumin (Figure 1). This modification is chemically added after the peptide is purified from fermentation.
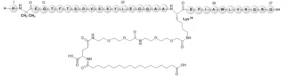
Semaglutide is the key therapeutic component responsible for Ozempic’s efficacy, while the formulation also contains excipients to enhance stability, bioavailability, and shelf life. Ozempic inactive ingredients include disodium phosphate, dehydrate (buffer agents), propylene glycol (tonicity agents), phenol (preservative), hydrochloric acid (pH adjustment), sodium hydroxide (pH adjustment), and water for injections (solvent). These ingredients are essential, especially considering that semaglutide is not refrigerated.
Besides its components, Ozempic and other semaglutide-based drugs can contain impurities formed during production and storage. Impurities of semaglutide might include by-products formed in fermentation by the host organism as well as in the recovery and purification process of semaglutide precursor, in the modification steps, and the purification process.
Why Controlling Semaglutide Impurities Matters: Safety, Efficacy, and Regulatory Standards
The presence of drug impurities in any pharmaceutical product raises concerns about patient safety, drug efficacy, and regulatory compliance.
During semaglutide development, identifying and measuring peptide-based impurities, which are structurally similar to the intended product, is a critical step. These impurities occur due to changes in the peptide sequence, such as unintended additions, deletions, oxidation, or shifts to D-form isomers. Such alterations can create inconsistencies in the final product, demanding strict monitoring and causing:
- Efficacy considerations: The therapeutic effectiveness of semaglutide depends on its chemical integrity. Impurities could reduce drug potency or alter pharmacokinetics, leading to variations in blood glucose control.
- Safety concerns: Some impurities have the potential to trigger immune responses, leading to side effects that could impact Ozempic immune system interaction. Previous cases with other GLP-1 peptide agonists, such as the clinical trial discontinuation of taspoglutide, demonstrated that even small modifications in peptide structure can lead to severe reactions, such as anaphylaxis.
- Regulatory compliance: Both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines for controlling peptide-related impurities. This includes setting maximum allowable limits for specific impurities. Any newly identified peptide impurity exceeding 0.5% must undergo immunogenicity assessment to prevent adverse immune responses.
The Role of Advanced Analytical Methods in Detecting Impurities in OZEMPIC
Semaglutide-related impurities arising from the rDNA technology synthesis process, such as diastereoisomers, truncated sequences, amino acid substitutions, oxidation, and reduction, can pose significant challenges in manufacturing Ozempic. Since minor sequence alterations can hinder effective separation using standard reversed-phase high-performance liquid chromatography (HPLC), advanced detection methods, such as HLPC coupled to mass spectrometry (HPLC-MS) play a crucial role in ensuring semaglutide’s purity and compliance with global regulatory standards.
Recent advancements in peptide synthesis technology and the expanding use of synthetic peptide drugs have spurred interest in developing generic versions of therapeutic peptides, particularly for drugs derived from recombinant DNA technology. Advanced detection methods are key players in ensuring the safety and efficacy of these alternatives.

In 2021, the FDA issued guidelines for Abbreviated New Drug Applications (ANDAs) on highly purified synthetic peptides from rDNA-based drugs, followed by the EMA’s 2023 draft on synthetic peptide development and manufacturing. Both regulatory frameworks stress the need to demonstrate structural and functional equivalence between generic candidates and their reference drugs. The primary amino acid sequence is especially crucial, as it directly influences therapeutic efficacy, necessitating rigorous comparison with the reference product.
For peptides exceeding 20 amino acids, peptide mapping is recommended to ensure sequence accuracy and quality control. Beyond verifying sequence accuracy, peptide mapping serves as a robust strategy to identify structural deviations, assess manufacturing consistency, and evaluate chemical stability over time. Traditionally, HPLC coupled with UV detection (HPLC-UV) has been employed for fragment analysis. However, challenges arise with structurally complex peptides, as HPLC-UV may fail to resolve or detect certain fragments, limiting its ability to achieve comprehensive sequence coverage.
Ultra-Performance Liquid Chromatography coupled with High-Resolution Mass Spectrometry (UPLC-HRMS) stands as a powerful alternative to achieve full sequence coverage.
Aware of the need to apply advanced analytical methods for peptide analysis, AMSbiopharma has developed a mapping peptide service using UPLC-HRMS. We offer advanced peptide analysis services focusing on quality control and ensuring compliance with regulatory standards.
Don’t hesitate to contact us if you have any questions regarding our analytical services. Our experts will be willing to help you and solve your doubts.
References
- European Medicines Agency. Ozempic: EPAR – Public assessment report [Internet]. Amsterdam: EMA; 2022 [cited 2025 Jan 30]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/ozempic-epar-public-assessment-report_en.pdf
- Kim SH, Kim SS, Kim HJ, Park EJ, Na DH. Peptide mapping analysis of synthetic semaglutide and liraglutide for generic development of drugs originating from recombinant DNA technology. J Pharm Biomed Anal. 2025 Jan 17;256:116682. doi: 10.1016/j.jpba.2025.116682
- Lee G. The battle for billions: understanding the Ozempic patent landscape. Columbia Science and Technology Law Review [Internet]. 2024 Nov 26 [cited 2025 Jan 30]. Available from: https://journals.library.columbia.edu/index.php/stlr/blog/view/653
- Lowe D. Compounded and counterfeit semaglutide [Internet]. Science.org; 2023 [cited 2025 Jan 30]. Available from: https://www.science.org/content/blog-post/compounded-and-counterfeit-semaglutide
- Pradhan, R.; et al. Glucagon-Like Peptide 1 Receptor Agonists and Risk of Anaphylactic Reaction Among Patients With Type 2 Diabetes: A Multisite Population Based Cohort Study. Am. J. Epidemiol. 2022, 191(8), 1352–1367. doi: 10.1093/aje/kwac021.


