In bioanalytical workflows, biomarkers serve as quantifiable proxies for biological processes, disease states, or therapeutic responses. Still, the rapid evolution of drug discovery and clinical development has transformed biomarkers from useful indicators into critical decision-making tools. Their application spans every stage of pharmaceutical R&D, from early target validation to clinical endpoint measurement, helping bridge the gap between laboratory findings and patient outcomes. As translational research becomes increasingly central to therapeutic innovation, the ability to identify, measure, and interpret biomarkers in biotechnology is now one of the most powerful drivers of successful drug development.
This article examines the role of biomarkers in modern bioanalysis, explores detection methods and validation strategies, and highlights emerging trends that are shaping their use in translational research and clinical development.
What Are Biomarkers? Types, Examples, and Bioanalytical Significance
Before examining their applications, it is essential to understand what biomarkers are and why they are useful. Biomarkers are biological indicators used to measure physiological or pathological processes or responses to therapeutic interventions. They may be molecules such as proteins, RNA, metabolites, or even digital signals derived from wearable devices and sensors. The main types of biomarkers include:
- Diagnostic biomarkers are used to detect or confirm the presence of a disease (for example, troponin levels in myocardial infarction).
- Monitoring biomarkers track the evolution of a disease or the effect of a treatment over time, such as blood pressure in hypertension or LDL cholesterol in cardiovascular prevention.
- Pharmacodynamic or response biomarkers signal whether a drug is engaging its target or producing the desired biological effect, like reduced glucose levels after antidiabetic therapy.
- Predictive biomarkers identify which patients are more likely to respond to a treatment. For example, HER2 expression in breast cancer guides trastuzumab therapy.
- Prognostic biomarkers estimate the likely disease course or outcome regardless of treatment, such as specific genetic profiles that indicate aggressive tumor behavior.
- Safety biomarkers warn of potential toxicity, as seen in elevated liver enzymes signaling hepatotoxicity.
- Susceptibility or risk biomarkers reveal predisposition to developing a condition before symptoms appear, such as genetic variants linked to cardiovascular risk.
Common biomarker examples include biomarkers in blood, such as C-reactive protein (CRP) for inflammation, biomarkers of kidney disease like creatinine, and biomarkers of rheumatoid arthritis, such as anti-cyclic citrullinated peptide antibodies. Beyond traditional categories, molecular approaches have introduced protein biomarkers (e.g., HER2 in breast cancer) and RNA biomarkers (e.g., BCR-ABL fusion transcripts in chronic myeloid leukemia). These markers are used to guide diagnosis but also to inform therapeutic decisions and enable real-time monitoring of disease progression.
A comprehensive list of biomarkers is continuously expanding as omics technologies advance. This complexity makes robust bioanalytical method validation essential to ensure reliability, reproducibility, and regulatory compliance of biomarkers in drug development workflows.
Role of Biomarkers in Translational Research and Drug Development
Biomarkers play a foundational role in translational research. They bridge the mechanistic understanding gained in preclinical models with clinical endpoints in patients. By translating molecular changes into measurable outcomes, they reduce uncertainty in drug development, accelerate go/no-go decisions, and improve the probability of clinical success.
Their applications extend across the entire R&D continuum:
-
- In target identification and validation, biomarkers confirm that a molecular pathway is both implicated in disease and modifiable through therapeutic intervention.
-
- During preclinical studies, they measure pharmacokinetics/pharmacodynamics (PK/PD) effects, allowing researchers to verify that a compound interacts with its intended target.
-
- In early clinical development, they provide early evidence of efficacy and safety, guiding dose selection and patient stratification.
-
- In late-stage trials, biomarkers can act as surrogate endpoints, offering earlier insight into clinical benefit and potentially supporting accelerated approval.
Integrating biomarkers accelerates development timelines and reduces the risk of costly late-stage failures, making them vital tools for efficient pharmaceutical innovation.
Bioanalytical Methods for Biomarker Detection: From Sampling to Validation
Accurate biomarker detection technology is central to their utility in research and clinical development. Bioanalytical workflows must capture molecular signals across diverse matrices, from plasma and tissue to cerebrospinal fluid, while maintaining sensitivity, specificity, and reproducibility.
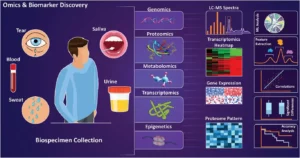
Figure 1. Biocompositional analysis of various biosamples. Source: Haghayegh F, et al. Revolutionary Point-of-Care Wearable Diagnostics for Early Disease Detection and Biomarker Discovery through Intelligent Technologies. Adv Sci (Weinh). 2024 Sep;11(36):e2400595.
The typical biomarker analysis pipeline involves:
- Sampling and pre-analytical handling: correct collection, stabilization, and storage of biological samples are crucial to prevent degradation or signal loss.
- Detection platforms: techniques include ELISA, multiplex immunoassays, quantitative PCR for nucleic acids, and advanced LC-MS for proteomic profiling.
- Data processing and quantification: high-throughput platforms require robust computational tools for signal normalization, calibration, and quality control.
- Validation: following regulatory guidelines for bioanalytical method validation, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) guidance, ensures assay accuracy, precision, linearity, and robustness.
As highlighted in recent scientific reviews, bioanalytical success increasingly depends on integrating automation, high-resolution instrumentation, and digital processing tools to reduce variability and enhance throughput. These advances enable more comprehensive bioanalysis of protein biomarkers, RNA biomarkers, and metabolites, strengthening the reliability of translational research data.
Ultimately, a well-validated bioanalytical workflow ensures that biomarker measurements are clinically meaningful. This alignment between analytical quality and biological interpretation is what allows biomarkers to serve as trusted indicators in drug development, regulatory evaluation, and personalized medicine.
Current Trends in Biomarker Analytics: Automation, Digital Tools, and Personalized Medicine
Biomarker analytics is entering a new phase shaped by automation, digitalization, and data integration. These advances are increasing analytical precision, scalability, and clinical relevance, particularly in the context of personalized medicine.
Automation now underpins much of modern bioanalysis, improving reproducibility and throughput through robotic sample handling and standardized workflows. Combined with advanced biomarker detection technology, these systems enable simultaneous analysis of multiple protein biomarkers and RNA biomarkers, reducing variability and accelerating discovery.
At the same time, digital transformation is reshaping data interpretation. Artificial intelligence and machine learning are being applied to multi-omic datasets, revealing hidden correlations and identifying new predictive and prognostic biomarkers. The rise of digital biomarkers, collected from wearable devices and mobile sensors, extends biomarker monitoring beyond the lab, offering continuous, real-world physiological insights.
Together, automation, AI, and connected technologies are transforming biomarker research into a truly translational discipline. By linking high-quality analytical data with individualized patient information, these innovations are paving the way for faster development, smarter trial design, and more precise, patient-centered therapies.

At AMSbiopharma, we deliver tailored bioanalytical solutions founded on cutting-edge science and regulatory best practices. Supporting pharmaceutical and biotech companies through method development, robust validation, and compliant testing, we enable confident implementation of biomarker-driven research programs. Partnering with AMSbiopharma means access to precision, reliability, and responsive collaboration essential for driving pharmaceutical innovation forward.
References
Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018 Feb;243(3):213-221. doi: 10.1177/1535370217750088
Cockburn JG, Mariappan V, Loke MF, Al-Maleki AR, Muttiah B, Vellasamy KM, et al. Integrative research: Current trends and considerations for biomarker discovery and precision medicine. Microbe. 2025;7(100368):100368. doi: 10.1016/j.microb.2025.100368
European Medicines Agency. ICH M10: Bioanalytical Method Validation – Scientific Guideline [Internet]. Amsterdam (The Netherlands): EMA; [cited 2025 Oct 10]. Available from: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline
Hendrikse NM, Llinares Garcia J, Vetter T, Humphreys AJ, Ehmann F. Biomarkers in Medicines Development-From Discovery to Regulatory Qualification and Beyond. Front Med (Lausanne). 2022 Apr 26;9:878942. doi: 10.3389/fmed.2022.878942
Moein MM, El Beqqali A, Abdel-Rehim M. Bioanalytical method development and validation: Critical concepts and strategies. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Feb 1;1043:3-11. doi: 10.1016/j.jchromb.2016.09.028
U.S. Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry [Internet]. Silver Spring (MD): FDA; [updated May 2018; cited 2025 Oct 10]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry


