 Preclinical studies of in vivo efficacy and safety requirements are an essential part of any drug development project. Key experimental aspects obtained with animals are intended to be used as a guide for the later complete trial design in order to obtain preliminary information regarding the experimental candidate’s toxicity, safety, or dosing strategy.
Preclinical studies of in vivo efficacy and safety requirements are an essential part of any drug development project. Key experimental aspects obtained with animals are intended to be used as a guide for the later complete trial design in order to obtain preliminary information regarding the experimental candidate’s toxicity, safety, or dosing strategy.
Various animal models (mainly rodents) have been used in-house to test the potential of drug candidates in an early stage. Our professional scientists will go along with you during this initial phase by adapting and customizing our processes to your specific needs: planning and design of the project, selection of the animal model, formulation of the product, dosing and routes of administration, etc.
General toxicology services:
The effect of any compound depends on the dose and the duration; therefore, toxicology services include acute and subacute studies to fulfill our customer´s demands.
Acute studies are intended to evaluate the effects after a single or multiple administration, usually immediately or within 24 h. A pre-defined fixed-dose procedure can be established by administering increasing or decreasing dose levels in order to assess the lethal or nonlethal toxicity; or a staircase design (up-and-down method) can be used, highly recommended to reduce the number of animals used in research, by dosing animals on sequential intervals with higher or lower doses depending on the survival.
Subacute studies are designed to determine the effects up to a minimum of 4 weeks. Test substance is administered regularly at a specific time. In these studies, lower dose levels are used to ensure that the animals tolerate the dose during the study.
Safety studies:
Histological evaluation: Tissues and organs can be collected so to provide insights into the damage or lesions caused by the experimental compound. Macroscopic and microscopic ultrastructural observation of abnormalities can be accomplished after previous studies so to provide insights on the toxicological effects of the novel candidate.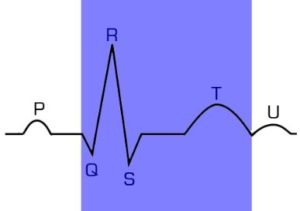
QT interval: The electrocardiogram evaluation (ECG) is a highly valuable tool for assessing the cardiac electrical function, so changes or modifications of the ECG waves and intervals are particularly important to determine the safety of the experimental compounds. The QT interval of the ECG represents the duration of ventricular depolarization and subsequent repolarization; therefore, it is widely used as indicator for drug cardiotoxicity. Preliminary electrophysiologic in vitro studies are required to determine potential hERG inhibition of drug candidates. Potassium efflux through the hERG-encoded channel is a key factor in the repolarization phase of ventricular myocytes, and inhibition of the hERG current indicates a prolongation of the QT interval. Further confirmation of these results can be accomplished with in vivo experiments, where additional safety parameters such as blood pressure, cardiac biomarkers or histological damage evaluation can be performed to complement results.
Cellular damage evaluation:
In vitro toxicological evaluation is one of the first steps on drug discovery processes. Cytotoxicity assays are a fast way to obtain cost-efficient vital information of your novel candidate, due to the low compound consumption.
Cell viability assays are carried out by our scientists according to our customer´s needs, measuring activities related to cellular maintenance and survival. Tests focus on the use of colorimetric assays, such as MTT, which is reduced by NAD(P)H-dependent oxidoreductase enzymes to the violet-colored formazan, indicative of the viable cells present at a stablished end-point; or resazurine, which is non-toxic and permeable to cells, allowing a continuous monitoring of the reducing environment of living cells.

